
PharmaShots Weekly Snapshots (October 09–13, 2023)
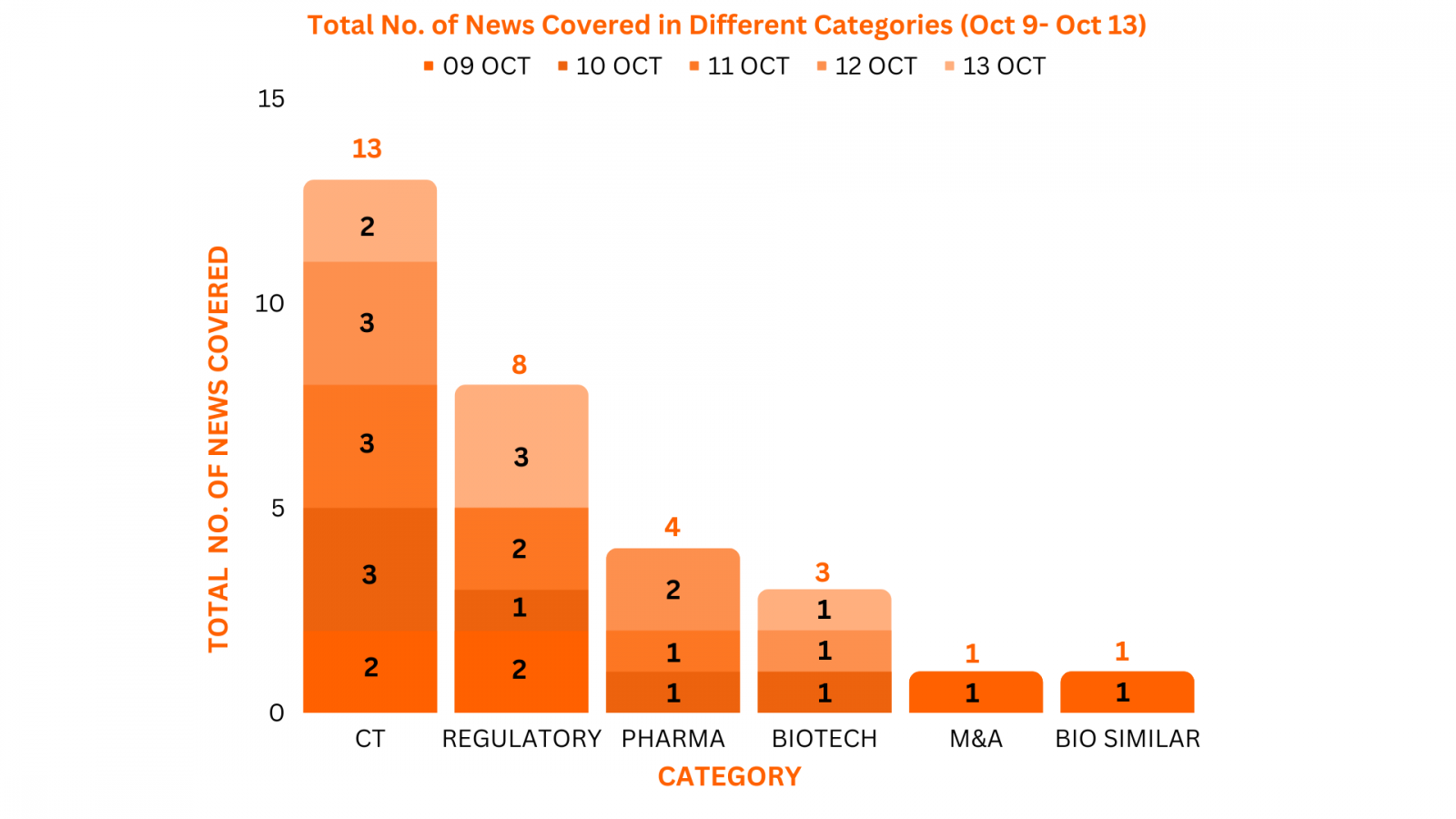
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, M&A, and biosimilar. Check out our full report below:

- The US FDA has approved Novartis’ Cosentyx (secukinumab) as the first intravenous formulation for Rheumatic Diseases
Read more: Novartis
- TFDA has approved CANbridge’ CAN108 (Livmarli) for the treatment of cholestatic pruritus in patients with Alagille syndrome in Taiwan
Read more: CANbridge
- The US FDA has approved Foundation Medicine’ FoundationOne CDx for Eli Lilly's Retevmo to treat solid tumors
Read more: Foundation Medicine
- The US FDA has granted ODD to SELLAS’ SLS009 for the treatment of Acute Myeloid Leukemia, based on the P-I study
Read more: SELLAS
- The US FDA has granted FTD to ImmPACT Bio’ IMPT-514 for Refractory Lupus Nephritis and Systemic Lupus Erythematosus
Read more: ImmPACT Bio
- The US FDA has approved Pfizer’s Braftovi (encorafenib) + Mektovi (binimetinib) or BRAF V600E-mutant metastatic non-small cell lung cancer, based on the P-II trial (PHAROS)
Read more: Pfizer
- The EMA’s CHMP has adopted a positive opinion recommending approval of Santhera’ Agamree for DMD patients aged ≥4yrs
Read more: Santhera
- Kyverna Therapeutics Receives the US FDA’s IND Clearance to Initiate the P-I/II Study of KYV-101 for the Treatment of Scleroderma
Read more: Kyverna Therapeutics

- Janssen highlighted P-IIIb study (ESCAPE-TRD) results of Spravato (esketamine nasal spray) for treatment-resistant major depressive disorder showed that patients achieved PHQ-9-defined remission
Read more: Janssen
- Innovent dosed the first patient of Efdamrofusp Alfa (IBI302) in the P-III clinical study (STAR) for neovascular age-related macular degeneration
Read more: Innovent
- Ventyx Biosciences highlighted P-II trial results of VTX002 for moderate-to-severely active ulcerative colitis showed that 28% of patients on 60mg dose & 24% on 30mg dose achieved the 1EPs of clinical remission at 13wk.
Read more: Ventyx Biosciences
- AnaptysBio highlighted P-III trial (GEMINI-1) results of Imsidolimab (Il-36r) for Generalized Pustular Psoriasis which met its 1EPs & achieved rapid clearance of pustulation, erythema & scaling through 4wk.
Read more: AnaptysBio
- Roche highlighted P-III studies (BALATON) & (COMINO) results of Vabysmo (faricimab) for the treatment of retinal vein occlusion which showed early & sustained vision improvement
Read more: Roche
- Merck highlighted P-III trial (KEYNOTE-671) results of Keytruda for Resectable Stage II, IIIA or IIIB non-small cell lung cancer met its dual 1EPs of OS
Read more: Merck
- Genentech highlighted P-III study (OCARINA II) results of Ocrevus for the treatment of Multiple Sclerosis showing that Ocrevus (SC) was non-inferior to IV infusion
Read more: Genentech
- Merck reported 5year results from the P-II OLE trial of Evobrutinib for Relapsing Multiple Sclerosis which showed sustained clinical efficacy & safety with no evidence of clinical worsening
Read more: Merck
- Incyte highlighted positive 52-week P-IIb trial results of Povorcitinib (INCB54707) for extensive nonsegmental vitiligo showed mean percentage total body & facial depigmentation improvement from baseline
Read more: Incyte
- AbbVie highlighted P-III (Measure Up 1), (Measure Up 2), and (AD Up) studies results of Rinvoq for Atopic Dermatitis at 32nd EADV Congress 2023 achieved the co-1EPs of improvement in skin clearance measured by an EASI 75 & vIGA-AD 0/1 at 16wk.
Read more: AbbVie
- BMS highlighted 3yr. results from the P-III (POETYK PSO) LTE trial of Sotyktu (deucravacitinib) for moderate-to-severe plaque psoriasis showed a consistent safety profile with no increases in the rates of AEs or serious AEs over time
Read more: BMS
- Eli Lilly highlighted P-III study (VIVID-1) results of Mirikizumab for the treatment of Crohn's Disease met the co-primary i.e., patients achieved clinical response at 12wk. & clinical remission at 52wk.
Read more: Eli Lilly
- AbbVie reported P-IIb study results of Rinvoq (upadacitinib) for Non-Segmental Vitiligo met the 1EPs of percent change from baseline in F-VASI at 24wk. with the 11 & 22mg doses
Read more: AbbVie

- BMS to acquire Mirati Therapeutics for ~$5.8B. The latest acquisition strengthens and diversifies BMS’ oncology portfolio
Read more: BMS and Mirati Therapeutics

- The US FDA has approved Bio-Thera Solutions’ Tofidence (IV), the first biosimilar to reference Actemra for moderately to severely active RA, polyarticular JIA & systemic JIA
Read more: Bio-Thera Solutions

- Biocon & Juno Pharmaceuticals collaborated to commercialize Liraglutide to Treat Type 2 diabetes and Obesity in Canada
Read more: Biocon & Juno Pharmaceuticals
- TaiGen & YSP collaborated to develop new drugs in Malaysia and Singapore. YSP will be responsible for the NDA and market sales within the authorized region
Read more: TaiGen & YSP
- Astria Therapeutics & Ichnos Sciences collaborated for the OX40 portfolio in Atopic Dermatitis and other allergic and immunological diseases
Read more: Astria Therapeutics & Ichnos Sciences
- Indivior & Alar collaborated to develop and commercialize ALA-1000 and future buprenorphine-based LAI product candidates
Read more: Indivior & Alar

- BioMap & Sanofi collaborated to co-develop AI modules & advance drug discovery for biotherapeutics using BioMap’s AI platform
Read more: BioMap & Sanofi
- EpimAb Biotherapeutics & Almirall collaborated to develop bispecific antibodies for up to three undisclosed target pairs
Read more: EpimAb Biotherapeutics & Almirall
- MediLink Therapeutics & BioNTech collaborated to develop next-generation anti-cancer antibody-drug conjugate
Read more: MediLink Therapeutics & BioNTech
Related Post: PharmaShots Weekly Snapshots (October 03–06, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














